Research Article
Optimization of Claudin-5 and ICAM-1 protein detection by using capillary-based immunoassay method in human brain endothelial cells
Nurul Farhana Jufri*, Tharsini Salyam, Farah Wahida Ibrahim, Mazlyzam Abdul Latif and Asmah Hamid
Programme of Biomedical Science, Center for Toxicology and Health Risk Studies, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur.
* Correspondence
Nurul Farhana Jufri
Programme of Biomedical Science, Center for Toxicology and Health Risk Studies, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur.
nurulfarhana@ukm.edu.my
Tel: +603-9289 7057
Received: 11 March 2021; Revised: 4 May 2021; Accepted: 1 June 2021; Published: 9 June 2021
DOI: https://doi.org/10.28916/lsmb.5.1.2021.80
ABSTRACT
Background: Human brain endothelial cells (HBECs) are part of the blood-brain barrier (BBB). BBB acts as a barrier to control the passage of molecules or materials from the blood into the brain. Identification of specific proteins changes in their expressions that are related to disease state is important in order to understand the disease mechanism involving brain vasculature. To achieve that, the techniques involve in identifying the proteins of interest must be optimized prior to further investigation. Methodology: In this study, identification of Claudin-5 in HBEC lysates was tested using different sample preparation techniques such as; 1) reducing with Dithiothreitol (DTT) and non-reducing conditions; 2) denaturing by heating at 95°C for 5 minutes or 70°C for 20 minutes and 3) protein loading at 3 and 4 µg. The samples were then subjected to an automated capillary-based immunoassay, Jess. Results and Discussion: The results showed that HBEC samples loaded at 4 µg and heated for 5 minutes at 95°C with DTT produced clearer and intense bands for Claudin-5 identification compared to the other set ups. As reducing condition and denaturing by heated at 95°C for 5 minutes conditions demonstrated good results, the conditions were used to identify ICAM-1 expression at different protein loading (3 and 4 µg). The result demonstrated that HBEC samples heated for 5 minutes at 95°C with DTT and loaded at 4 µg produced a good detection for ICAM-1. Conclusion: These optimized conditions could be served as a standard procedure for further identification of Claudin-5 and ICAM-1 proteins in HBEC using a capillary immunoassay instrument.
Keywords: Brain endothelial cells; inflammatory protein; protein expression; reducing agent and tight junction proteins
INTRODUCTION
Protein detection and identification could be conducted via several methods such as western blot or enzyme-linked immunosorbent assay. However, with evolving technologies, other protein identification techniques have been introduced. One of the alternative approaches is by using capillary-based immunoassays. The protein lysate sample, together with the separation matrix, stacking matrix, antibody and reagents are loaded onto a plate and the components are aspirated in each capillary. Voltage is then applied to the capillaries to enable proteins to be separated according to their molecular weight. Once the separation is completed, ultraviolet (UV) light immobilizes the proteins to the capillary wall and an immunoprobing process can take place with a specific primary antibody, followed by the application of horseradish peroxidase-conjugated secondary antibody. Finally, a chemiluminescence substrate is added to enable the protein migration to be recorded (Nelson et al., 2017). This method requires less amount of protein with a high throughput that is less laborious compared to the conventional western blot. An optimization strategy to identify the best conditions involved in the sample preparation prior the actual analysis using real samples is important to ensure a successful and reliable protein detection.
Normal brain function is guarded by a homeostatic mechanism, the blood-brain barrier (BBB), that strictly restricts the movement of substances from the periphery to the central nervous system (CNS). The endothelial cells lining the cerebral blood vessels are the core anatomical structure of the BBB. The tight junctions (TJ) are the main component that links endothelial cells together and any insults to the TJ will result in a compromised BBB function. BBB integrity is maintained by the TJ that are composed of transmembrane proteins, such as occludin, claudin and accessory proteins namely zona occludens (Abbott et al., 2010). Claudin-5 is the most enriched type of TJ protein in the brain endothelial cells and its dysfunction has been implicated in neurodegenerative and neuroinflammatory disorders (Greene et al., 2019). Intercellular Adhesion Molecule 1 (ICAM-1) is constitutively present on endothelial cells, and its expression is increased by proinflammatory cytokines and invokes a range of proinflammatory responses (Lawson & Wolf, 2009). The increase of ICAM-1 expression is essential for the adhesion of leukocytes to the inflamed tissues during inflammation (Wang et al., 2014). Claudin-5 and ICAM-1 expressions were implicated in pathogenesis involving brain vascular such in Alzheimer’s disease that is characterized by a greater prevalence of collapsed or degenerated endothelium and severe impairment of the BBB transport system. Decreased Claudin-5 demonstrated high vascular permeability due to the disruption of the BBB integrity in neurodegenerative patients (Su et al., 1999). Meanwhile, ICAM-1 acts as inflammatory marker and it is involved in the neuroinflammation process of neurodegenerative diseases (Grammas, 2011).
Lysosome dysfunction could be caused by gene mutation or aging (Peng et al., 2019). Several chemicals have been identified as lysosome inhibitor such as ammonium chloride that can be used to induce lysosome dysfunction. Ammonium chloride has the ability to penetrate the lysosome resulting in an increase in the lysosomal pH thus prevent the normal functioning of the lysosome (Misinzo et al., 2007). Identification of Claudin-5 and ICAM-1 expressions in endothelial cells through lysosome inhibited in vitro model is crucial in understanding the mechanism of neurodegenerative diseases. These could elucidate the roles of lysosomes in the blood-brain barrier that is implicated in neurodegenerative disease. The knowledge could be used for future therapeutic development. The application of capillary-based immunoassay technique to detect these proteins is relatively new, therefore, identification of the optimal conditions is part of the preliminary work that need to be done before the actual research is conducted in control and treated group using the standardized conditions respectively.
MATERIALS AND METHODOLOGY
Cell culture
Human brain endothelial cells-5i (HBEC-5i) were grown in T75 flasks in Dulbecco Modified Eagle’s Medium/Ham’s F-12 (Nacalai Tesque Kyoto Inc, Japan) added with 1% penicillin and streptomycin (Nacalai Tesque Kyoto Inc, Japan) and 10% fetal bovine serum (FBS) (HyClone, Utah South America). All cell lines were grown in 5% CO2 at 37°C and regularly screened for progressive growth and free from contamination. Passage used were between 5-7.
Protein lysate preparation
Flasks containing HBEC were treated with 59 mM ammonium chloride (Sigma Aldrich, USA) for 12 hours except for the control group. The protein samples or cell lysates were collected after the treatment. Cells were washed twice with cold PBS and lysed with cold RIPA lysis buffer (VWR Chemicals, USA). A scraping method under cold conditions was used to harvest the cells, which were then transferred into microcentrifuge tubes. Cells were then vortexed three times for homogenization and were centrifuged at 13,500 rpm, 4°C for 15 minutes. The supernatants were collected for further analysis.
Protein quantification
The microplate assay procedure was performed using the BCA Protein Assay kit (Thermo Scientific, USA). A total of 10 µL of standards and cell lysate samples in triplicate were pipetted into microplate wells with 200 µL of BCA working reagent and the plate was then mixed thoroughly on a plate shaker for 30 seconds. The plate was then covered and incubated at 37°C for 30 seconds. Then, the plate was cooled to room temperature and absorbance was measured at 562 nm (Bio-Rad iMark, USA).
Protein samples preparation
All reagents and samples were prepared according to the manufacturer's protocol. A standard pack reagent comprising of ladder, dithiothreitol (DTT) and 5X fluorescent master mix was prepared. The samples were 12 hours ammonium chloride treated samples (T), control samples (C) and the biotinylated ladder.
Experimental group
Control sample (untreated) was grouped accordingly to three groups: i) heat block temperature and timing (95°C for 5 minutes and 70°C for 20 minutes), ii) the presence or absence of 400 mM DTT reagent, and iii) two different amounts of protein loads (3 and 4 µg). Control sample was used to detect Claudin-5 optimal expression condition. Due to sample limitation, baseline conditions for temperature and denaturing conditions detected from control sample was used to detect ICAM-1 on HBEC sample treated with ammonium chloride.
Protein expression by Jess
Protein expression was measured by an automated capillary-based immunoassay instrument known as Jess by Protein Simple of Bio-Techne, United States. The samples were vortexed, centrifuged for five seconds and were placed on ice. Next, primary antibodies; ICAM-1 mouse monoclonal IgG (Santa Cruz Biotechnology, United States), Claudin-5 mouse monoclonal IgG (Santa Cruz Biotechnology, USA) and secondary antibody m-IgGk HRP conjugated (Santa Cruz Biotechnology, USA) were prepared with a dilution of 1:10. Subsequently, the chemiluminescent agent was freshly prepared by mixing luminol-S and peroxide in 1:1 ratio, vortexed for homogenization and kept on ice. Lastly, a total of 10 µL of prepared samples and 10 µL of reagents were loaded into the assay plate according to the plate design. The ladder was placed in the first row, followed by the antibody diluent, primary antibody, streptavidin-HRP, secondary antibody, luminol peroxide and 500 uL wash buffer in the top three rows of buffer wells. The assay plate was then centrifuged at 1000 × g for five minutes at room temperature. Finally, protein separation, electrophoresis and immunodetection analysis were run on the Jess automated capillary system. Sample peaks and densitometric bands were viewed and analyzed from the derived data by using Compass Software (Protein Simple, USA).
RESULTS
Figure 1 (i) shows the bands that correspond to the expression of Claudin-5 using control samples that were heated in different temperature and reducing condition, A: 95°C for 5 minutes in reduced condition, B: 70°C for 20 minutes in reduced condition, C: 95°C for 5 minutes in non-reduced condition and D: 70°C for 20 minutes in non-reduced condition. Sample A showed the intense band intensity (highest band intensity) at 23 kDa amongst all of the samples. Meanwhile, sample D demonstrated the faintest band (lowest band intensity). Figure 1 (ii), the band intensity values were further measured by chemiluminescence peak height and the result confirmed that sample A had the highest band intensity followed by B, C and D.
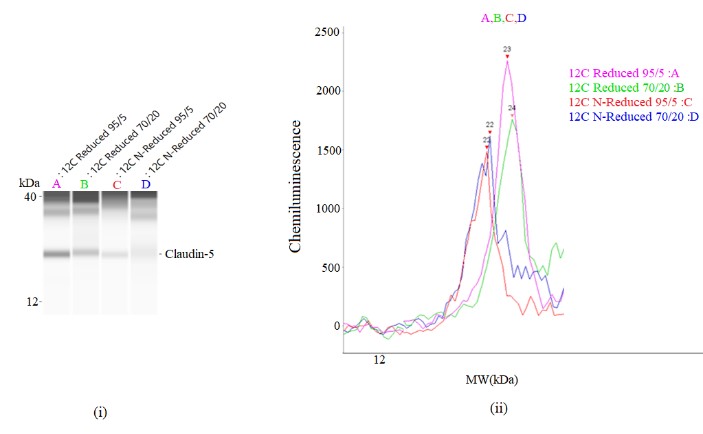
Figure 1: (i) Densitometry image of Claudin-5 expression on human brain endothelial cells by capillary imm
